Harnessing the Power of Self-Assembly for Nanoscale Design
As an innovative bottom-up approach, self-assembly—the spontaneous organization of molecules into stable, well-defined structures—offers an exciting paradigm for creating nanoscale templates under ambient conditions or at the liquid-solid interface. Self-assembled molecular networks (SAMNs) on surfaces are a perfect illustration of this principle in action. In our research, we go beyond simply analyzing the structural properties of these networks. We delve into the fascinating dynamics within self-assembled layers, exploring how molecular design and other factors influence their behavior. By examining the time scales and forces that drive this organization, we aim to unlock new ways to control molecular interactions and tailor material properties at the nanoscale. Additionally, we seek to activate these dynamics on demand, applying these principles to the investigation of molecular motors.
Decoding the Impact of Molecular Chirality on Surfaces
Understanding how molecular chirality influences the interaction and organization of molecules on surfaces is a critical frontier in scientific research, with far-reaching implications across various fields. Chirality—the unique “handedness” of molecules—plays a pivotal role not only in fields like chromatography but also in cutting-edge phenomena such as the chiral-induced spin selectivity (CISS) effect.
The study of chirality at the molecular level opens new pathways for breakthroughs in nanotechnology and catalysis. By uncovering how chirality shapes molecular organization and interactions on surfaces, we aim to unlock new tools for controlling chemical reactivity, and creating advanced materials with novel electronic properties.
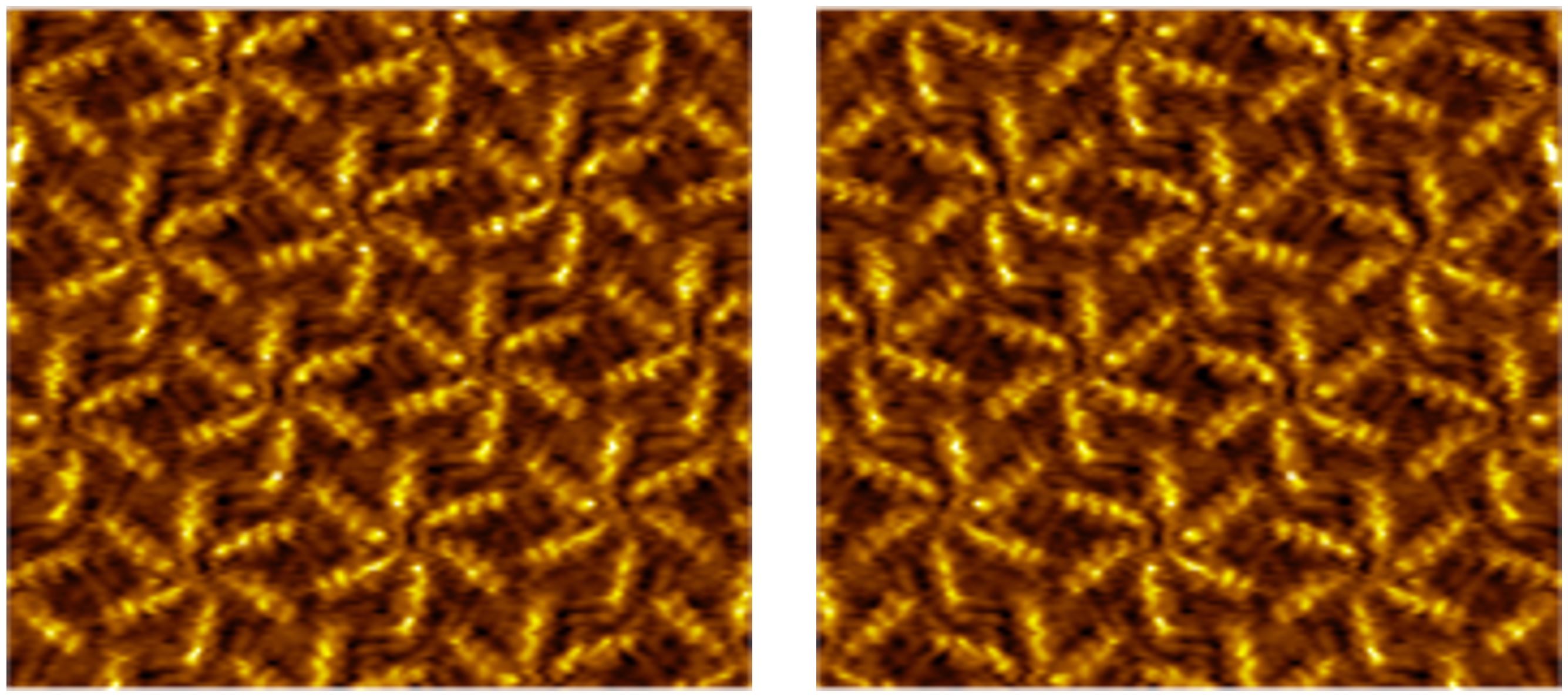
From On-Surface Reactions to New Materials
Scanning probe microscopy offers a powerful window into the world of surface reactions, providing submolecular resolution—even at the liquid-solid interface. Our research focuses on investigating carefully selected reactions on surfaces, with a particular emphasis on using scanning tunneling microscopy (STM) to visualize these processes in exquisite detail.
Our motivation is three-fold: First, we aim to understand the critical role of the substrate in surface-confined chemical reactions, as certain reactions occur exclusively on surfaces. Second, we leverage surfaces as platforms for the formation of novel molecules, such as 2D polymers, which cannot be synthesized in solution. Finally, by conducting these reactions directly on surfaces, we gain the ability to tailor and modify the properties of the surfaces themselves, opening up exciting possibilities in material science and nanotechnology.
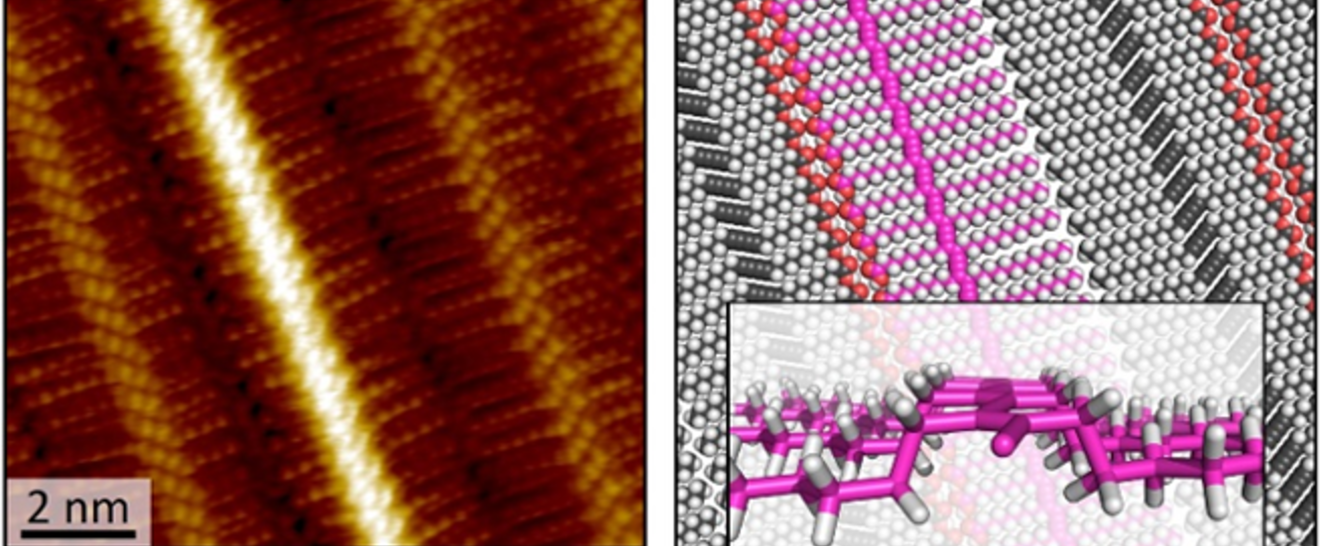
Chemistry in Nanoconfined Spaces
We explore the fascinating process of molecular self-assembly within precisely defined nanoscale areas, known as “nanocorrals.” These nanocorrals—created with varying size, shape, and orientation—are formed on covalently modified graphite surfaces through scanning probe nanolithography, or "nanoshaving." Our research has unveiled a remarkable discovery: when nanoshaving is performed at the liquid−solid interface, the orientation of self-assembled monolayers is significantly influenced. By carefully aligning the nanoshaving direction with the substrate’s symmetry axes, we can control the alignment of the supramolecular structures within these corrals. Moreover, the unique geometric and kinetic constraints imposed by nanocorrals of different shapes and sizes provide new insights into the dynamics of self-assembly at the nanoscale, and reveals previously unknown phases.
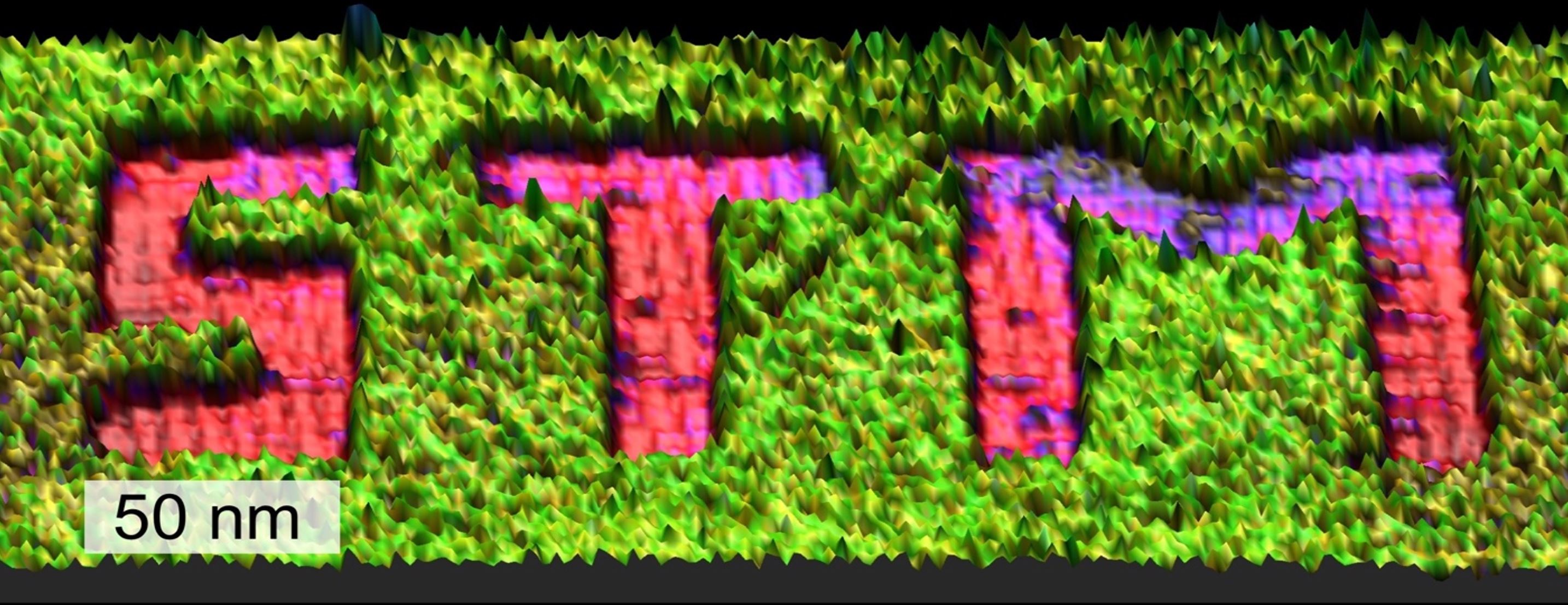
2D Materials: from Modification to Application
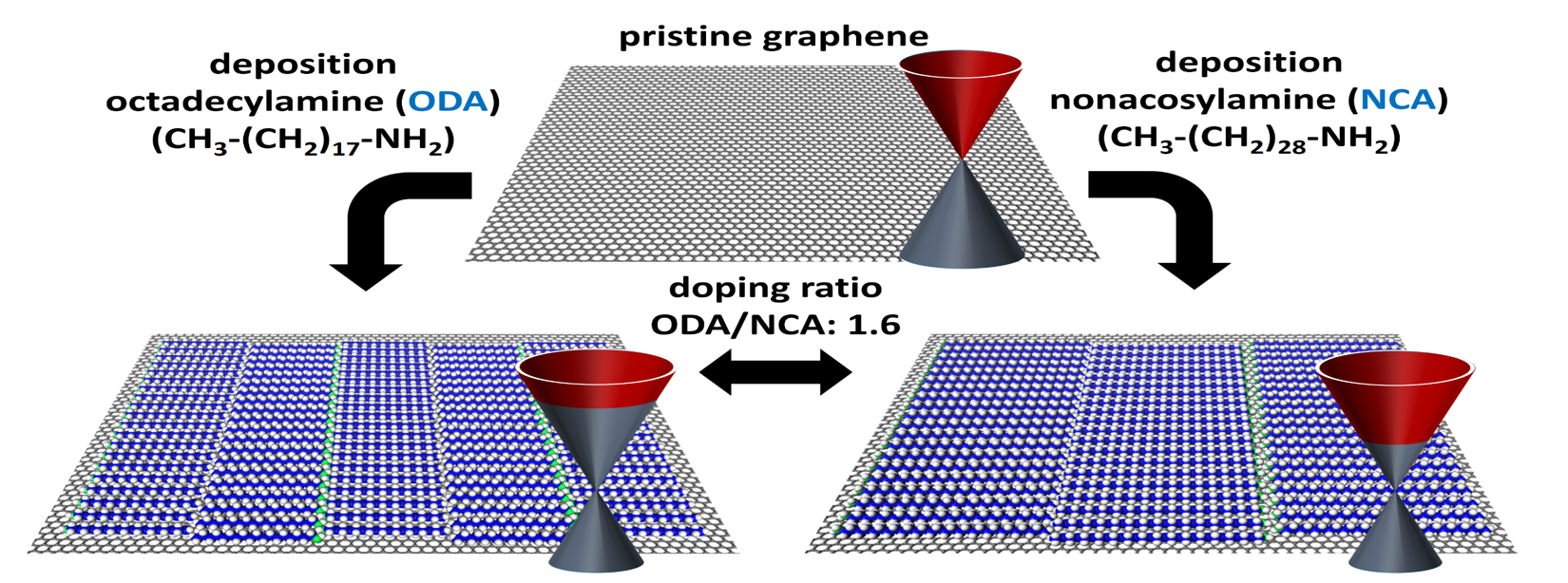
Our research focuses on altering the properties of 2D materials like MoS₂, graphene, and beyond through both covalent and non-covalent functionalization strategies. Building on our expertise in molecular self-assembly on surfaces—especially on graphite—we are now applying these concepts to harness the extraordinary potential of graphene. One exciting approach involves the functionalization of graphene using physisorbed self-assembled molecular networks (SAMNs), a technique that offers precise and uniform control over doping. This strategy opens up new pathways for tailoring the electronic and chemical properties of 2D materials, pushing the boundaries of what’s possible in nanotechnology and materials science.
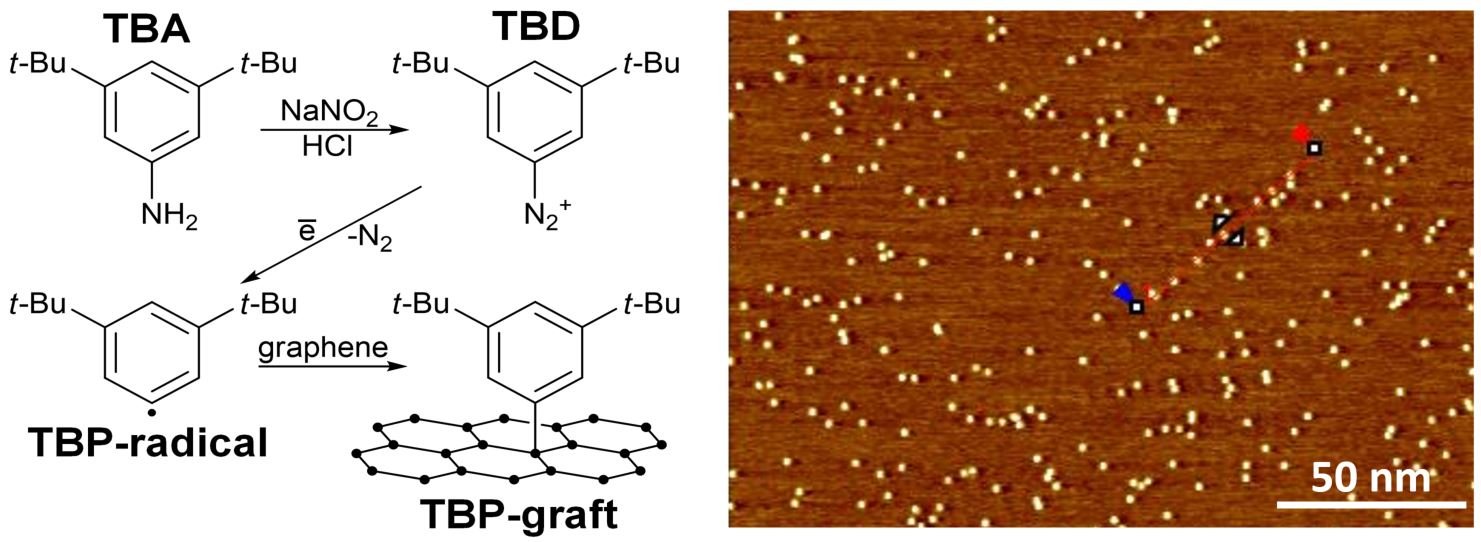
In addition to non-covalent methods, covalent functionalization strategies also offer a powerful way to modify the properties of 2D materials. Recently, we developed a protocol for the controlled functionalization of graphene and MoS₂ using radical chemistry, carefully avoiding unwanted multilayer formation.
What’s truly fascinating is that scanning tunneling microscopy (STM) provides an unparalleled nanoscale view of the process, revealing each individual grafting site in stunning detail. This allows us not only to control the degree and density of functionalization, but also to achieve spatial precision at nearly the atomic level—paving the way for new possibilities in material design and nanoengineering. We use such nanoengineered substrates for sensing and catalysis.
From Designer Proteins to DNA Origami
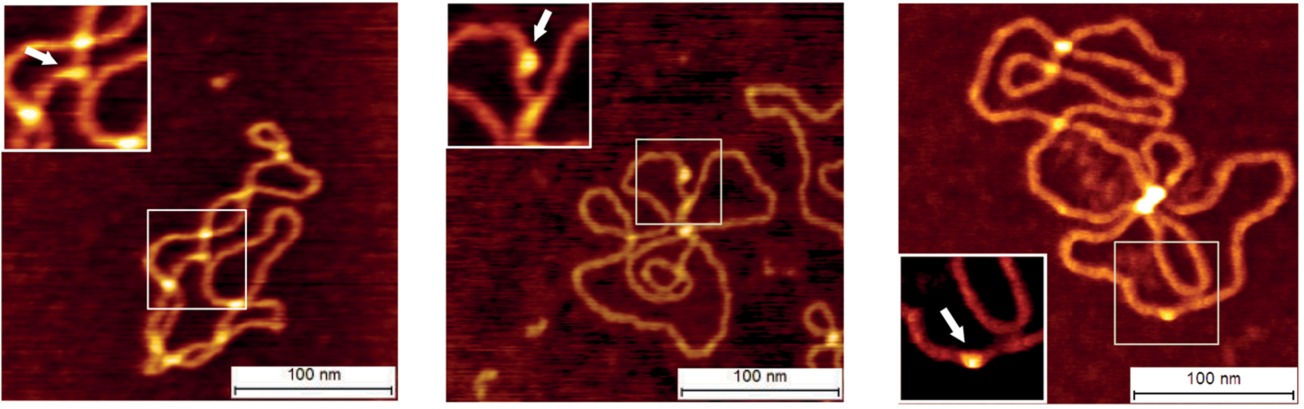
We also delve into the intricate world of biological materials on surfaces. Our current research is centered on the fascinating self-assembly of designer proteins and DNA origami, unlocking new possibilities in nanobiotechnology. At the heart of our work is atomic force microscopy, enabling us to visualize and manipulate these structures with unparalleled precision.


